I. 4.1 FREE RADICAL HALOGENATION
The addition of Cl or Br added to an alkane in the presence of UV light (hv) or heat
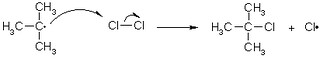
1. INITIATION: homolytic cleavage of halide into 2 radicals (with single, unpaired e-)
2. PROPAGATION:
a) Free radical collides with alkane, forming H-X and alkane radical (R·)
(1) The Hs are usually on tertiary carbons (Cs stabilized by neighbors)
b) The tertiary radical then reacts with a molecular halogen, forming alkyl halide
c) Halogen radical is regenerated
3. TERMINATION: occurs when any reactive radicals combine with each other (no more propagation will occur)
OR
OR
4. INHIBITION: Free radical can be inhibited by oxygen to form a less reactive alkyl peroxy radical
R· + O═O ⇌ R—O—O·
B. SUMMARY OF FREE RADICAL HALOGENATION OF ALKANES MECHANISM
1. Initiation → increase in # of free radicals
2. Propagation → no net change in # of free radicals
3. Termination → decrease in number of free radicals
4. Inhibition → slows down reaction by reducing the amt of reactive radical intermediate (reversible)
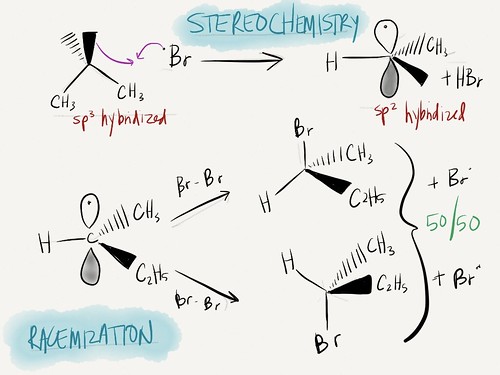
C. STEREOCHEMISTRY OF FREE-RADICAL HALOGENATION
1. After abstraction (halide stealing hydrogen) in propagation step, carbon radical rehybridizes
a) Goes from sp3 to sp2, with the lone electron in an unhybridized p orbital
b) In next step of propagation, C—X bond can form on either side, leading to enantiomers (50-50 mix)
D. STABILITY OF ALKYL RADICALS
1. Stability similar to carbocations → 3º > 2º > 1º in terms of stability
a) 3º (tertiary) means there are 3 other carbons surrounding it
E. SELECTIVITY
 1. Take 2-methyl propane, for example
1. Take 2-methyl propane, for example
a) There are 9-3º and 1-1º Hydrogens, so you would expect a 9:1 ratio of 3º Cl to 1º Cl, but this is not the case
Selectivity = reactivity/(# of sites available)
Selectivity 3º = (reactivity at 3º)/(# of 3º sites) = 37/1 = 37/7 = 5.3
Selectivity 1º (reactivity at 1º)/(# of 1º sites) 63/9
2. **Bromination is much more selective than chorination (much prefers 3º carbons)
a) Just know that the reason for this is that chlorination is much more exothermic, so that makes bromination more slow and selective
II. 4.2 NUCLEOPHILIC SUBSITUTIONS
Replace a leaving group in an electrophilic substrate with a nucleophile
A. NUCLEOPHILES AND ELECTROPHILES
1. Nucleophiles – Species that have unshared (unpaired) electrons or π bonds, and frequently, a negative or partially negative charge (δ–)
a) Nucleus-seeking or nucleus-loving molecules
b) Electron pair donors
c) Lewis bases (because they donate electrons)
d) Ligand (think ligand sound like lye, which is basic)
2. Nucleophilicity – how strong a nucleophile is; trends include:
a) Increasing nucleophilicity as negative charge increases (obvious)
b) Increasing nucleophilicity going down a group
(1) Related to polarizability → more shielding, more electrons, more polarizable
c) Increasing nucleophilicity going right to left across a group (more shielding)
(1) Related to electronegativity → more electronegative means that the more stable an atom is with a negative charge, and less nucleophilic
3. Electrophiles – electron deficient species; have a positive or partially positive (δ+) charge
a) Electron-loving
b) Lewis acid (accept electron pair)
4. In all organic reactions (except free radical halogenation), nucleophiles are attracted to, and donate and electron pair, to electrophiles
a) When the electrophile accepts the electron pair (Lewis acid/Lewis base rxn), a new covalent bond is formed
B. THE SN2 MECHANISM (subsitution reaction)
1. Alkyl halide → alkyl halide with a different alkyl group (or other strong nucleophile)
a) Since halogens are very electronegative, they make good leaving groups
2. Step 1 (and only)
a) One halide attacks the electrophilic C at the same time the leaving group leaves
3. The attack must occur from the back side of the substrate
4. The Br—C bond forms as the I—C bond is broken in a single step
5. There is an inversion of configuration
6. Reaction rate = k
7. SN2 → s = subsitution, N = nucleophilic, 2 = bimolecular
8. Protic solvents, like water, need to be avoided
a) Aprotic (non H-bonding), polar substances (acetone) should be used; the polar nature allows the nucleophiles and leaving groups to remain dissolved
C. THE SN1 MECHANISM (subsitution reaction)
1. Alkyl halide → halogen replaced with something else (O—H or O—R)
2. The more stable the carbocation, the more likely a reaction will take place!
3. 2 Steps:
a) Halogen leaves, and 120º planar carbocation forms (3º most stable) → very slow, this is the rate-limiting step
b) Weak nucleophile attacks either side, resulting in 50-50 racemic mixture
4. Reaction rate = k[electrophile=]
5. Solvents – should use protic solvents (water, alcohol); they help stabilize the carbocation and also behave as the nucleophile
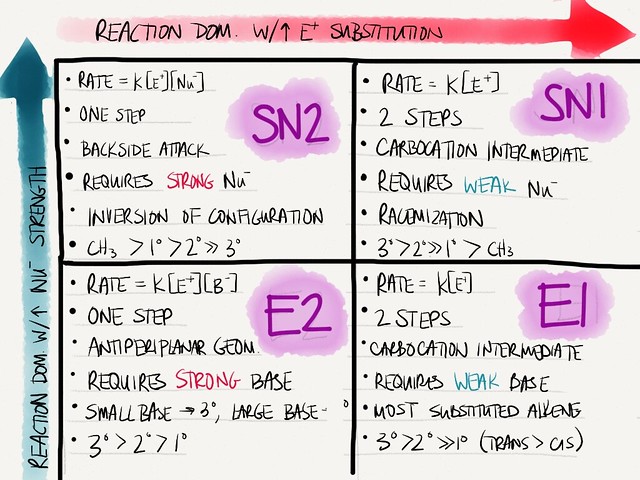
D. KEY FEATURES OF SN2 AND SN1 REACTIONS
1. You want to sit on a bench, but there is a hobo sleeping on it…
a) SN2 – kick the hobo off the bench
(1) You (nucleophile) form a bond to the substrate (bench) at the same time the hobo (leaving group) leaves
b) SN1 – wait for the hobo to leave, then sit on the bench
(1) The hobo (leaving group) leaves the bench (substrate), and you (nucleophile) form bond with the bench (substrate)
| FEATURES | SN2 | SN1 |
| Reactivity of substrate | CH3 > 1º > 2º >> 3ºMust be alkyl halide | 3º > 2º >> 1ºMust be alkyl halide |
| “Big barrier” | Steric hinderance | Carbocation stability |
| Stereochemistry | Complete inversion | Almost complete 50/50 (racemic) |
| Kinetics | Reaction rate = k[electrophile][nucleophile] | Reaction rate = k[electrophile] |
| Solvent | Polar, non-H-bonding solvents (aprotic) | H-bonding solvents (water, -OHs) |
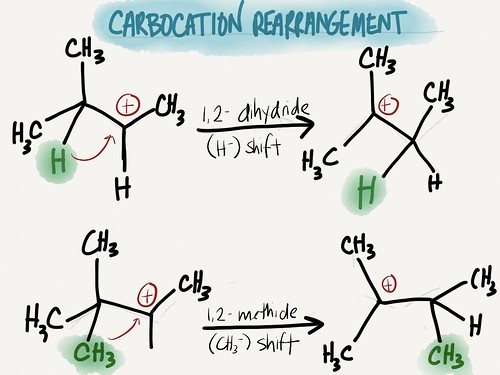
| Rearrangements | Not possible | Carbocation will rearrange to most stable if possible |
| Favoring conditions | Strong (charged), non-bulky nucleophile | Non-basic, weaker (noncharged) nucleophiles (often solvents act as nucleophile) |
E. SUBSITUTION REACTIONS WITH OTHER FUNCTIONAL GROUPS
1. Ethers – generally weak bases that are only reactive with strong acids
a) This seems kind of dumb (the first part)
2. Amines
a) Alkyl amines – N is bound to sp3 hybridized carbon
(1) Undergo rapid pyramidal inversion with little energy, so amines are not chiral!
b) Aryl amines – N is bonded to sp2 hybridized carbon in a ring
c) Can also be categorized as 1º, 2º, 3º, 4º (attached to 1 C, 2 C, 3 C, or 4 C)
3. Alkylation of Amines
a) Alkyl amines can participate in SN2 subsitution reactions by acting as a strong nucleophile (lone electron pair)
III. 4.3: ELIMINATION REACTIONS
Defined by the bonding changes that occur over the course of the reaction; 2 σ bonds are converted into a π bond
A. THE E1 MECHANISM – occurs via a 2-step mechanism
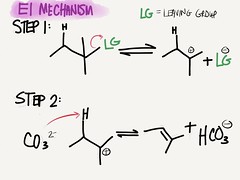 1. Step 1: the LG falls off, leaving carbocation
1. Step 1: the LG falls off, leaving carbocation
2. Step 2: a weak base then steals a proton of the C next to the carbocation, leaving behind the electrons to form a double bond
a) This can only happen if there is a H on the next carbon!
b) If there is more than 1 H to choose from, isomers form
(1) Trans isomers are favored over cis
(2) Can only occur if there is an H to steal
(3) Includes dehydration of alcohol reactions
c) Carbocation rearrangement – remember, most stable carbocations are 3º, so intermediate carbocations that are unstable will quickly rearrange to a more stable carbocation
B. THE E2 MECHANISM – 1 step mechanism
1. Step 1: strong base (usually OH or alkoxide ion) removes the β-hydrogen while the leaving group falls off → C-C double bond forms at the same time
a) Leaving group must be a halide
b) Works best if the proton removed is on the other side of the molecule as the LG, which makes the stereochemistry predetermined by the transition state
c) Base removes the proton from the most subsituted carbon if the base is small; if the base is bulky, it can’t get in and reach that proton, so it removes the most accessible β-proton
| FEATURES | E2 | E1 |
| Reactivity of substrate | 3º > 2º > 1º | 3º > 2º >> 1º (b/c of carbocation stability) |
| Stereochemistry | Bulky base → less substituted alkene, Small base → most substituted alkene | Most subsituted ═ form, trans > cis |
| Kinetics | Reaction rate = k[base][substrate] | Reaction rate = k[haloalkane] |
| Solvent | Polar, non-H-bonding solvents | H-bonding (protic) solvents (water, -Ohs) → stabilizes carbocation |
| Rearrangements | Not possible | Carbocation rearrangement |
| Favoring conditions | E2 > E1 with strong base; high temps favor elimination > substitution | Elimination >> substitution with weak base, high temps |
COMPARE
| FEATURES | SN2 | SN1 |
| Reactivity of substrate | CH3 > 1º > 2º >> 3º | 3º > 2º >> 1º (due to steric hinderance) |
| Stereochemistry | Complete inversion | Almost complete 50/50 (racemic) |
| Kinetics | Reaction rate = k[electrophile][nucleophile] | Reaction rate = k[electrophile] |
| Solvent | Polar, non-H-bonding solvents | H-bonding solvents (water, -OHs) |
| Rearrangements | Not possible | Carbocation will rearrange to most stable if possible |
| Favoring conditions | Strong, non-bulky nucleophile | Non-basic, weaker nucleophiles (often solvents act as nucleophile) |
C. SUMMARY OF SUBSITUTIONS AND ELIMINATIONS
| Requires strong base (nucleophile) | Requires weak base (nucleophile) |
| 1 step | 2 steps |
| Rate is dependent on substrate and base | Rate dependent only on substrate |
| Rearrangement occurs |
IV. 4.4: PROPERTIES OF ALCOHOLS
A. HYDROGEN BONDING
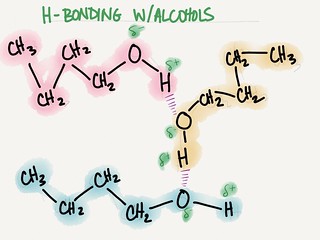 1. Consider the isomers n-butanol and diethyl ether:
1. Consider the isomers n-butanol and diethyl ether:
a) n-butanol → CH3—CH2—CH2—CH2—OH
b) diethyl ether → CH3—CH2—O—CH2—CH2—CH3
2. Difference in boiling points due to H-bonding (substantial δ- on H of hydroxyl and δ+ on O of hydroxyl)
B. HYDROGEN BONDING IN PHENOLS
1. Can cause intramolecular interactions (vs intermolecular)
2. This will ↓ interactions between molecules, which will ↓ boiling/freezing point!
C. ACIDITY
Determined by ease with which the alcohol can lose a proton
1. Relatively acidic because of large difference in electronegativity in O and H
2. If an alcohol is deprotonated, alkoxide ion is formed, which is pretty stable because the negative charge is located on a very electronegative ion (compare with a carbanion)
3. Acidity of phenols >> acidity of alcohols → due to resonance structures
a) Phenoxide ion very stable due to resonance
4. Electron-withdrawing substituents on phenols ↑ acidity because these further stabilize the negative charge through resonance
a) ↑ places for the (-) charge to delocalize to
5. Electron-donating substituents tend to destabilize the ion and ↓ the acidity of the substituted phenols
a) More electron density in the molecule means ↓ places for the (-) charge to delocalize
V. 4.5: FORMATION OF ALKYLE HALIDES: A SUMMARY
A. Notes about leaving groups:
1. Weak bases are the best leaving groups
2. High electronegativity makes a better leaving group
3. Large size makes better leaving group
4. Resonance of the remainder molecule increases a group’s ability to leave
B. Reaction of alcohols with phosphorus halides
CH3—CH2—CH2—OH + PBr3 → 3 CH3—CH2—CH2—Br + H3PO3
C. Reaction of Alcohols with Thionyl Chloride
CH3—CH2—OH + SOCl2 (phenol w/ N in it) → CH3—CH2—Cl + (phenol with NH+) + SO2(g)